
Jordan informed the Council that the appropriation for this year included a directive to the Public Health Service to reduce the funds spent on travel.

In a discussion of future meeting dates, Dr. The minutes from the September Council were approved without change.
Jordan introduced two visitors to the meeting, Winnie Stachelberg of OMB and Ms. Also introduced were Beverley Shenandoah, Elise Feingold, and Trenise West. She noted that the Center was pleased to have established a Personnel Office and introduced Mary Glynn and Cheryl Wild. Jordan introduced new members of the NCHGR staff. He briefed the Council on three issues that were discussed at the recent PAC meeting:ĭr. Watson reported on the "state of genome." He began with a report from the most recent meeting of the Program Advisory Committee. Others present for all or a portion of the meeting were:ĭr. Ivan Hernandez, Grants Management Specialist

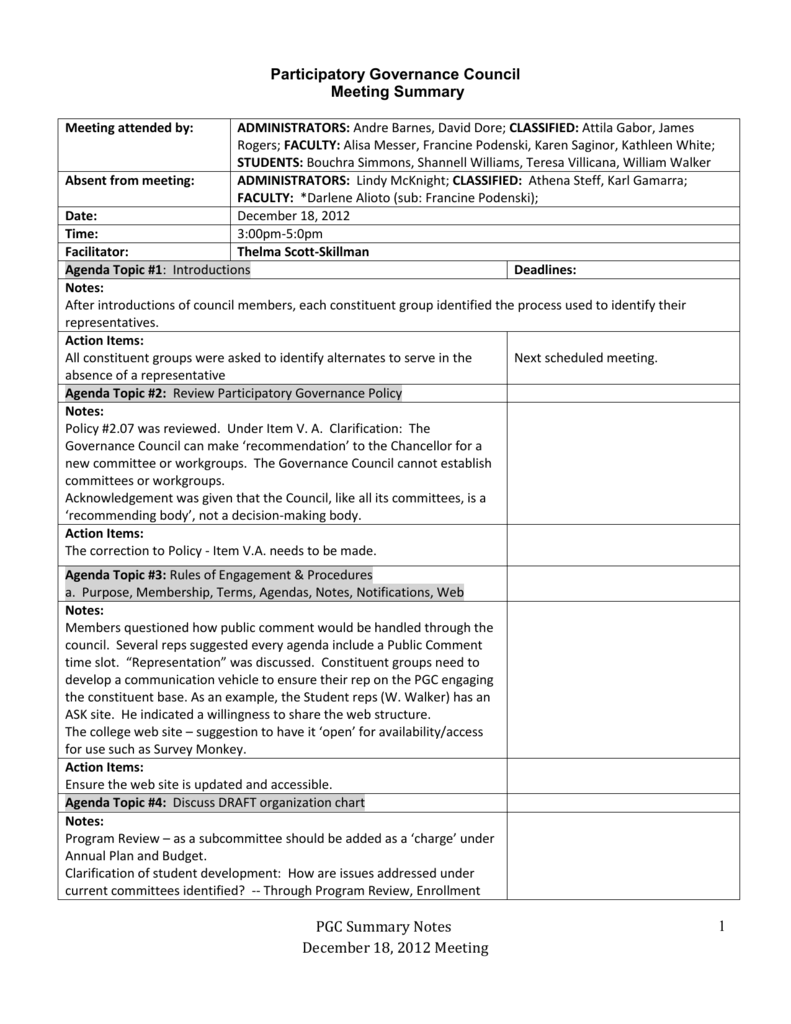
Mark Guyer, Assistant Director for Program Coordination Bettie Graham, Chief, Research Grants Branchĭr. Leslie Fink, Chief, office of Human Genome Communicationsĭr. Linda Engel, Chief, Office of Scientific Review Carol Dahl, Scientific Review Administrator, Office of Scientific Review This does not apply to "en bloc" actions.ĭr. Members are asked to sign a statement to this effect. David Benton Assistant to the Director for Scientific Data ManagementĪudrey Burwell, Grants Management Specialistįor the record, it is noted that to avoid a conflict of interest, Council members absent themselves from the meeting when the Council discusses applications from their respective institutions or in which a conflict of interest may occur. Midge Bajefsky, Grants Technical Assistantĭr. Staff of the National Center for Human Genome Research attending, in addition to Dr. The meeting was closed to the public for the review, discussion, and evaluation of grant applications. In accordance with the provisions of Public Law 92-463, the meeting was open to the public from 8:30 A.M. Elke Jordan, Deputy Director of the National Center for Human Genome Research (NCHGR), introduced the ad hoc consultants for this meeting, Dr. on January 27, 1992, at the Hyatt Regency Hotel, Bethesda, Maryland. The National Advisory Council for Human Genome Research was convened for its fourth meeting at 8:30 A.M. Issues raised − High Quotation, long duration, hourly mode of payment.ĭecision − The representatives were told to consult with their Management and report.Sample of Meeting Minutes for a Federal Advisory Committee (FACA)Ī Sample of Meeting Minutes for a Federal Advisory Committee (FACA) MeetingĪ Sample of Meeting Minutes NATIONAL ADVISORY COUNCIL Topic- Meeting with Hasta La Vista representatives at 6:00PMĪgenda at hand − Meeting with Hasta La Vista representatives Task List − task allotted and the respective allottee.įuture Meetings − the date and topic of the next meeting. Suggestions − made along with the names of the speakers. Issues raised − along with the names of the speakers. Topic − after two return keys Center-aligned.Īttendees − Name and designation (2 columns of a table).Ībsentees − name, roles, reasons for absenteeism. Name of the company − to the top-left of the page. Format of Minutes of MeetingĪ minutes of meeting normally includes the following elements − Their purpose is to record what actions have been assigned to whom, along with the achievements and the deadlines. They include the list of attendees, issues raised, related responses, and final decisions taken to address the issues. Also known as protocol or note, minutes are the live written record of a meeting.


 0 kommentar(er)
0 kommentar(er)
